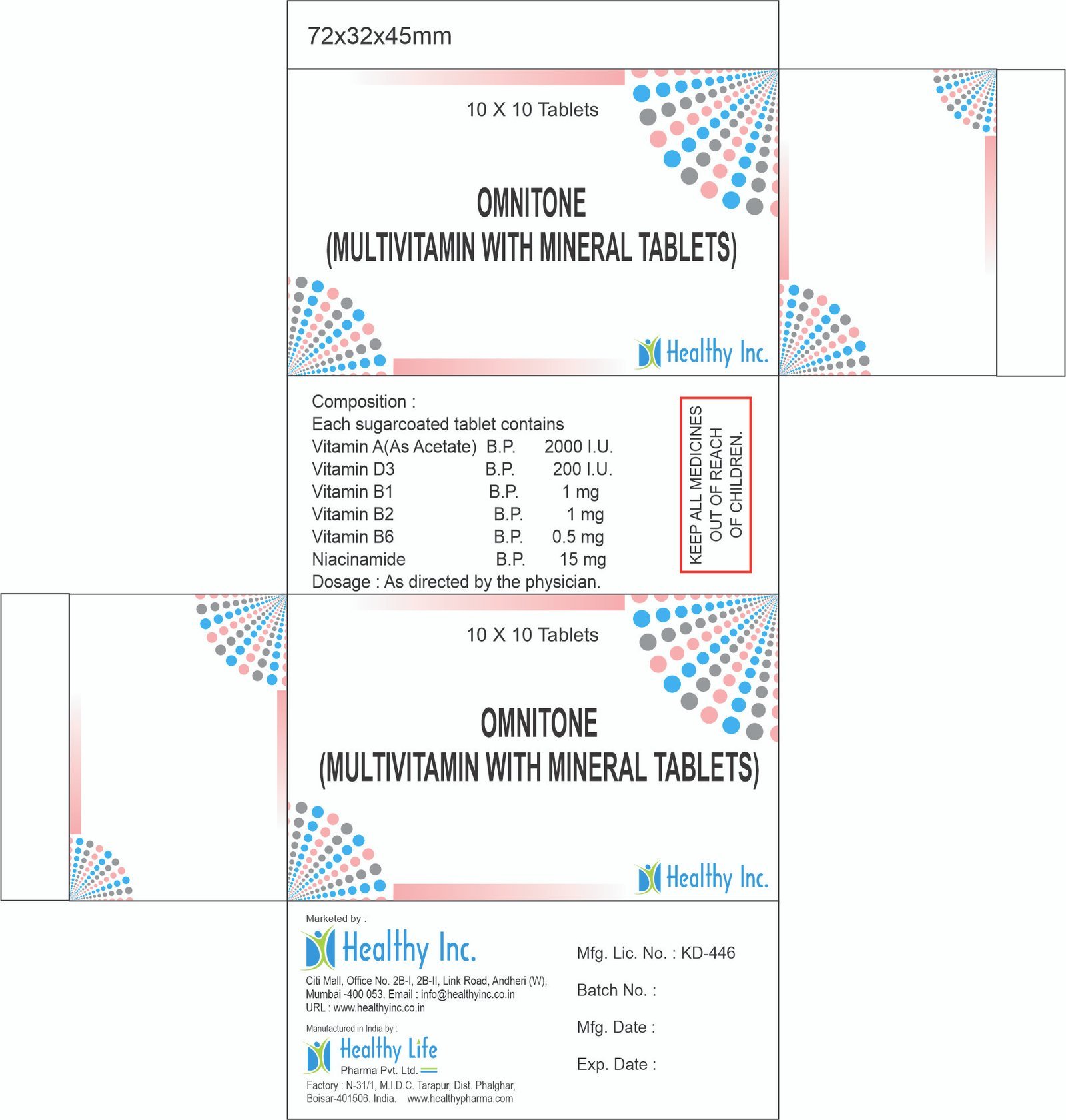
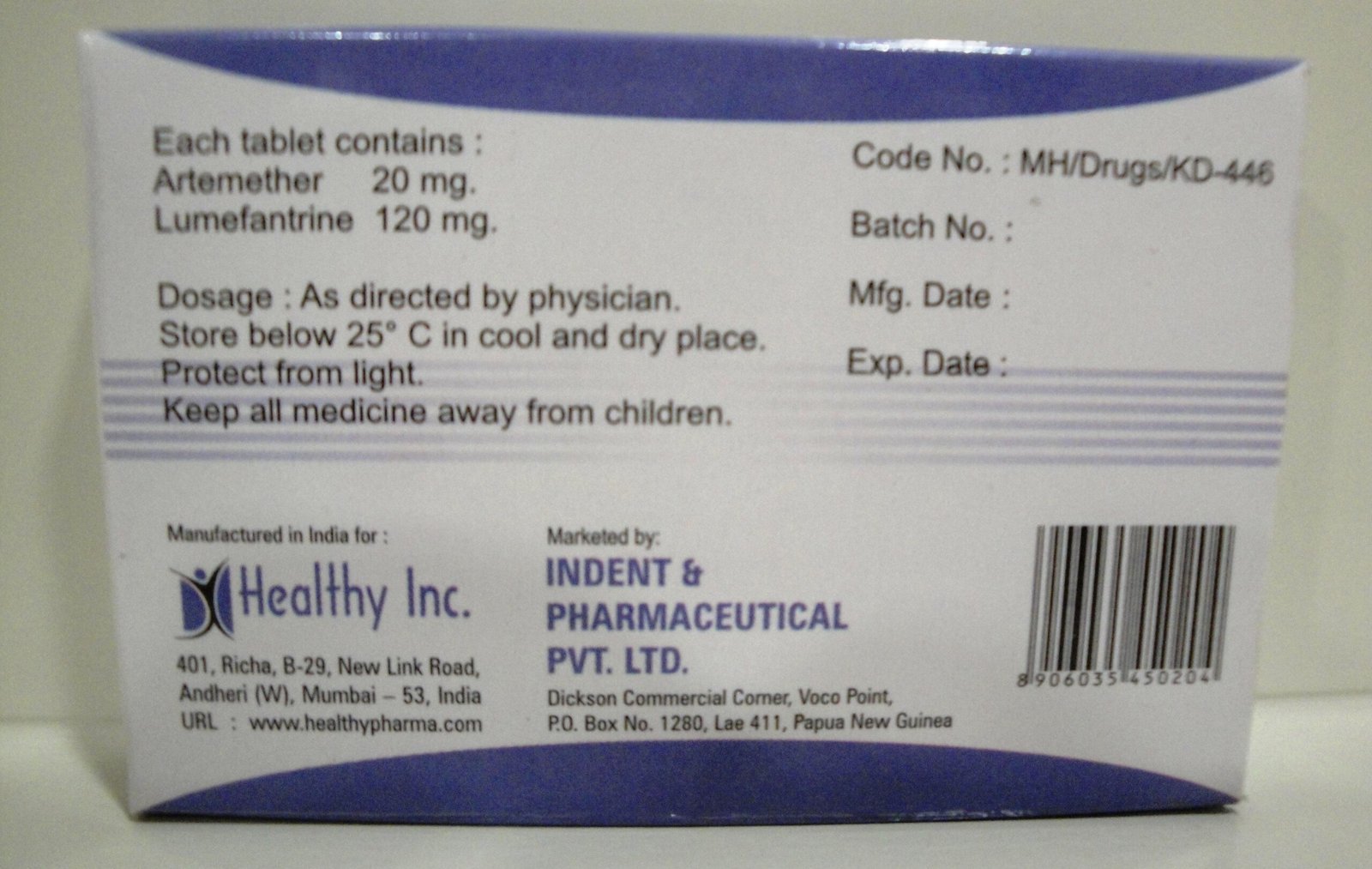
Description
Meropenem & Sulbactam Injection
Healthy Inc is a leading global supplier of advanced carbapenem combination therapies. We provide Meropenem & Sulbactam Injection (1.5 g), manufactured in dedicated, state-of-the-art WHO–GMP sterile facilities. This “Next-Generation” antibiotic is a critical export to intensive care units (ICU), specialized fever clinics, and government health ministries in Africa, LATAM, and Southeast Asia, specifically engineered to combat Multi-Drug Resistant (MDR) Gram-negative pathogens and ESBL-producing organisms.
Product Overview
This formulation combines Meropenem, an ultra-broad-spectrum carbapenem, with Sulbactam Sodium, a potent beta-lactamase inhibitor with unique intrinsic activity against certain non-fermenters.
The “Resistance Shield” Specialist:
- Synergistic Protection: While Meropenem is stable against many enzymes, the rise of Carbapenemases (like OXA-type) can threaten its efficacy. Sulbactam acts as a “decoy,” neutralizing beta-lactamase enzymes and restoring the potency of Meropenem against resistant strains.
- Acinetobacter Specialist: Unlike other inhibitors, Sulbactam has direct bactericidal activity against Acinetobacter baumannii. This makes the Meropenem-Sulbactam combination the preferred weapon against “Ventilator-Associated Pneumonia” caused by MDR Acinetobacter.
- Optimized PK/PD: The 2:1 ratio (1g Meropenem + 0.5g Sulbactam) is designed to maximize the time above the Minimum Inhibitory Concentration (MIC), ensuring clinical success in the most difficult-to-treat systemic infections.
Product Composition & Strength
We supply this product as a Sterile Lyophilized Powder for Injection in glass vials.
| Active Ingredient | Strength | Role |
|---|---|---|
| Meropenem USP/BP | 1000 mg (1 g) | Broad-Spectrum Carbapenem |
| Sulbactam Sodium USP/BP | Equivalent to 500 mg | Beta-Lactamase Inhibitor |
| Total Strength | 1.5 g (1500 mg) | Per Single Dose Vial |
*Pack Sizes: Single Vial with or without Sterile Water for Injection.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.20.19 (Medicaments containing other antibiotics) |
| Dosage Form | Lyophilized Powder for Infusion |
| Packaging | Type I Glass Vial with Flip-off Seal. |
| Storage | Store below 25°C. Keep Dry. Protect from light. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Dual-Powder Homogeneity: Blending Meropenem and Sulbactam requires precise environmental controls (Low Humidity < 30%) to prevent clumping and ensure that the ratio remains exact in every vial.
- Zero Cross-Contamination: Our facility utilizes dedicated air-handling units (AHUs) for carbapenem production to ensure zero contamination with other beta-lactams, ensuring safety for penicillin-sensitive patients.
Therapeutic Indications (Human Use)
Indicated for severe infections caused by multi-drug resistant organisms:
- Nosocomial Pneumonia: Including Ventilator-Associated Pneumonia (VAP).
- Complicated Urinary Tract Infections (cUTI): Including Pyelonephritis.
- Intra-abdominal Infections: Peritonitis and abscesses.
- Sepsis/Septicemia: Life-threatening systemic bacterial infections.
- Skin and Soft Tissue Infections: Complicated diabetic foot infections.
Dosage & Administration
Recommended Dosage (Strictly as per Critical Care Specialist):
- Route: Intravenous (IV) Infusion ONLY.
- Standard Dose: 1.5 g (one vial) administered every 8 hours.
- Administration Rate: To be infused over 15 to 30 minutes. In cases of severe resistance, extended infusion (over 3 hours) may be utilized to maximize efficacy.
- Renal Adjustment: Dose modification is essential in patients with CrCl < 50 ml/min.
Safety Warnings (CRITICAL):
- Seizure Risk: Although lower than Imipenem, Carbapenems can lower the seizure threshold. Use with caution in CNS disorders.
- Allergy: Contraindicated in patients with known hypersensitivity to Carbapenems or previous anaphylactic reactions to Penicillins.
- C. difficile: As a broad-spectrum antibiotic, it may lead to Clostridium difficile-associated diarrhea.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for Carbapenem combinations, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.






Reviews
There are no reviews yet.