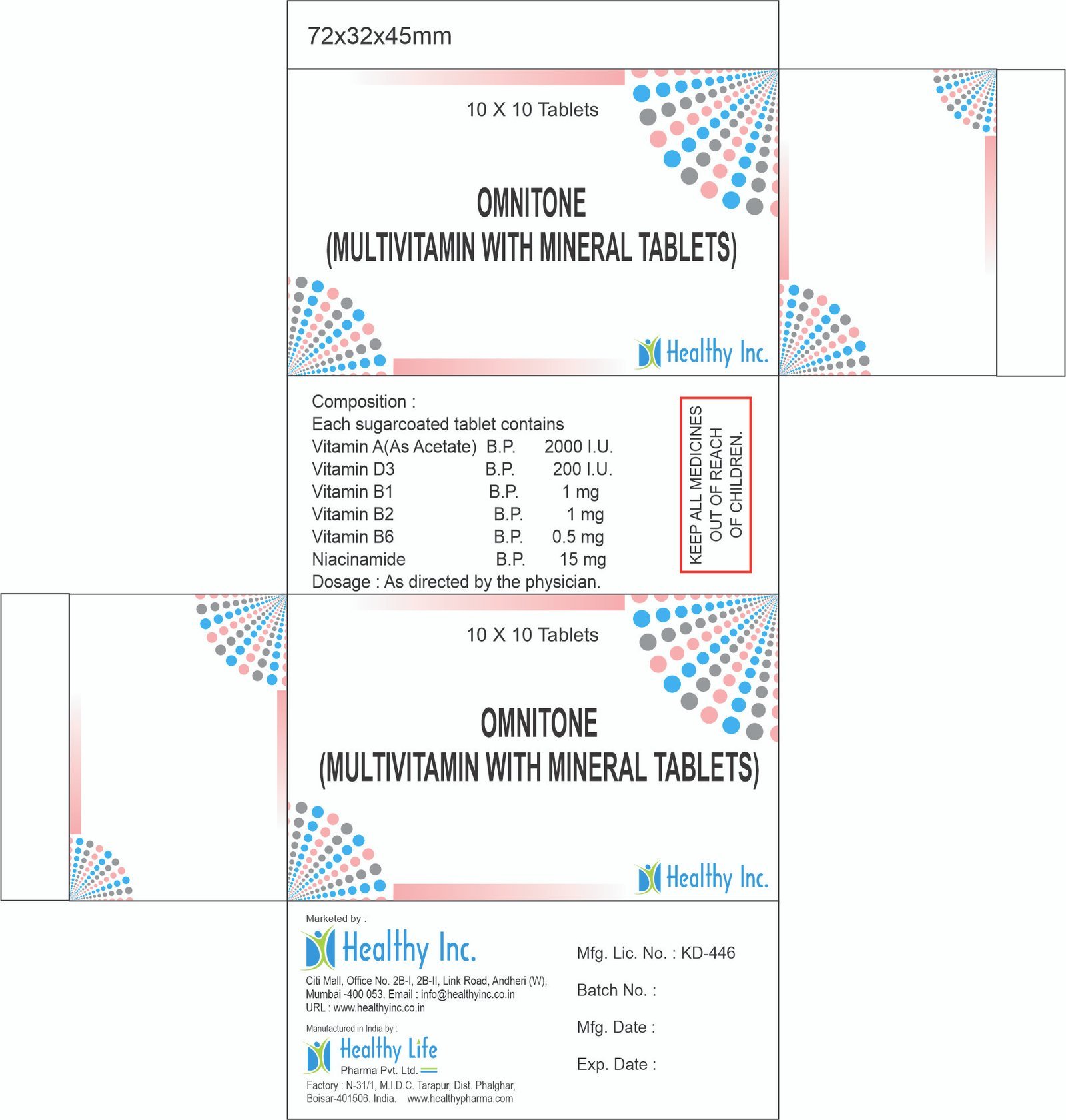
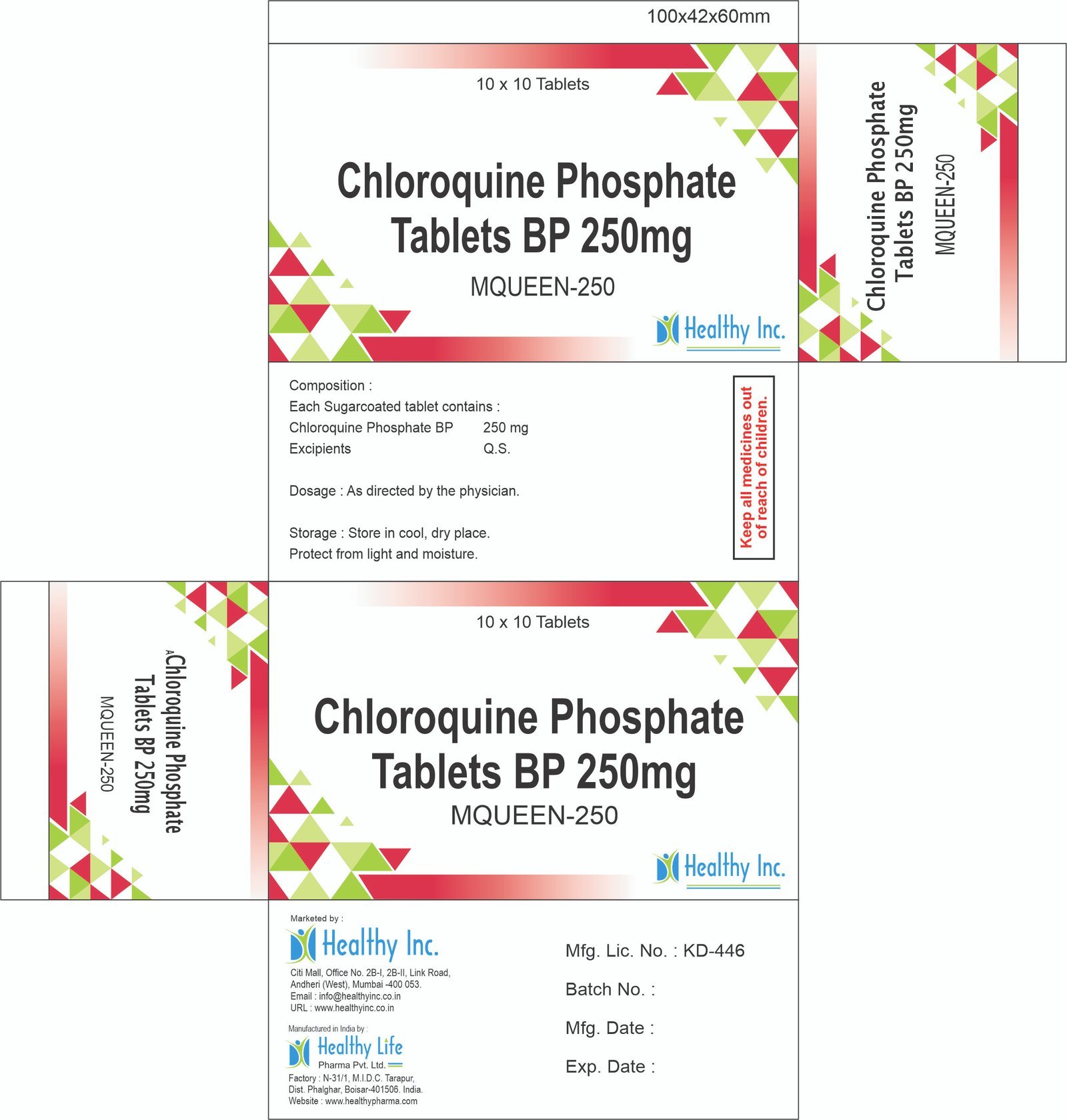
Description
Ceftizoxime Sodium Injection
Healthy Inc is a specialized global supplier and exporter of metabolically stable third-generation cephalosporins. We provide high-purity Ceftizoxime Sodium Injection (1g), sourced from WHO–GMP certified sterile injectable facilities. This “Anaerobic Specialist” is a top export to gastroenterology wards, gynecology clinics, and government health ministries in Africa, LATAM, and Southeast Asia, serving as a unique third-generation agent with potent activity against anaerobes and 100% unchanged renal excretion.
Product Overview
This formulation contains Ceftizoxime Sodium, a third-generation cephalosporin that distinguishes itself by not being metabolized in the body.
The “Metabolic Survivor” Specialist:
- 100% Unchanged Excretion: Unlike Cefotaxime (which is metabolized), Ceftizoxime is not metabolized at all. It is excreted 100% unchanged in the urine. This results in extremely high, potent concentrations in the urinary tract, making it the ultimate weapon for difficult UTIs.
- Anaerobic Activity: It possesses superior activity against anaerobic bacteria (including Bacteroides fragilis) compared to Ceftriaxone or Cefotaxime. This makes it an excellent single-agent choice for intra-abdominal infections and Pelvic Inflammatory Disease (PID).
- Gonorrhea Potency: It is highly effective against penicillinase-producing Neisseria gonorrhoeae (PPNG), often used as an alternative to Ceftriaxone.
Product Composition & Strength
We supply this product as a Sterile Dry Powder for Injection in glass vials. It contains approx. 2.6 mEq (60 mg) of sodium per gram.
| Active Ingredient | Strength | Primary Use |
|---|---|---|
| Ceftizoxime Sodium USP/BP | Equivalent to 1000 mg (1 g) Ceftizoxime | Severe Infection / Anaerobic |
| Ceftizoxime Sodium USP/BP | Equivalent to 500 mg | UTI / Gonorrhea |
| Excipients | None (Pure Sterile Powder) | – |
*Pack Sizes: Tray of 1 Vial, 10 Vials, or Box of 1/10 Vials with Water for Injection (WFI).
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.20.19 (Medicaments containing other antibiotics) |
| Dosage Form | Powder for Injection (Dry Fill) |
| Packaging | Type I Glass Vial (Sterile) with Flip-off Seal |
| Storage | Store below 25°C. Protect from Light. Reconstituted solution stable for 8 hours at room temp. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Sterile Crystallization: Ceftizoxime is highly water-soluble. We use advanced sterile crystallization techniques to ensure the powder remains free-flowing and chemically stable (white to pale yellow) without turning into a hard cake during storage.
- pH Balance: The formulation is naturally pH balanced (6.0 – 8.0) upon reconstitution, minimizing injection site pain without the need for additional buffers.
Therapeutic Indications (Human Use)
Indicated for the treatment of moderate to severe infections:
- Genitourinary Tract: Complicated UTIs (due to high urinary concentration) and Gonorrhea.
- Intra-abdominal Infections: Peritonitis and abscesses (covers aerobes and anaerobes).
- Pelvic Inflammatory Disease (PID): Effective against mixed flora involved in female pelvic infections.
- Septicemia: Gram-negative sepsis.
- Skin & Soft Tissue: Diabetic foot infections and burn wounds.
Dosage & Administration
Recommended Dosage (Strictly as per Physician):
- Route: Intravenous (IV) Infusion or Deep IM Injection.
- Adults: 1 g to 2 g every 8 to 12 hours.
- Life-Threatening Infections: 3 g to 4 g every 8 hours.
- Uncomplicated UTI: 500 mg every 12 hours.
- Gonorrhea: 500 mg Single IM dose.
- Renal Impairment: Dose reduction is mandatory if Creatinine Clearance < 50 ml/min.
- Reconstitution: Dissolve 1g vial in 10ml WFI. Shake well. CO2 is NOT released (unlike Ceftazidime).
Safety Warnings (CRITICAL):
- Penicillin Allergy: CONTRAINDICATED in patients with a history of anaphylaxis to beta-lactams.
- Transient Eosinophilia: Can occur with prolonged use.
- Diarrhea: Monitor for C. difficile colitis.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for Sterile Injectables, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.







Reviews
There are no reviews yet.