Description
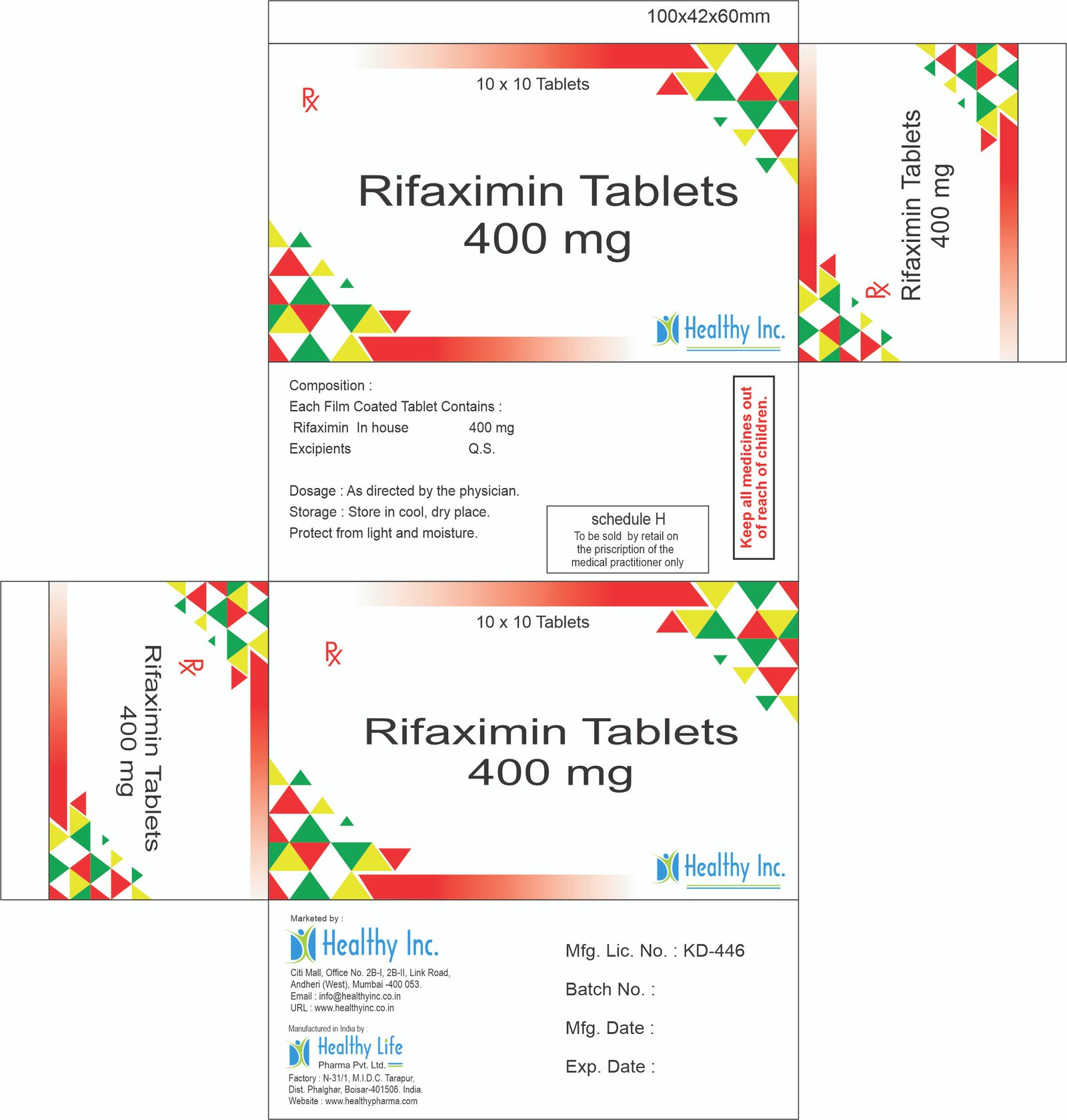
Rifaximin Tablets
Healthy Inc is a specialized global supplier and exporter of “Gut-Selective” antibiotics. We provide high-purity Rifaximin Tablets, sourced from WHO–GMP certified solid dosage facilities. This “Site-Specific” antibacterial is a top export to gastroenterology centers, liver clinics (Hepatology), and travel medicine providers in Africa, LATAM, and Southeast Asia, serving as the gold standard for treating Traveler’s Diarrhea, IBS-D, and preventing Hepatic Encephalopathy recurrences.
Product Overview
This formulation contains Rifaximin, a semi-synthetic derivative of Rifamycin with a unique pharmacokinetic profile.
The “Non-Systemic” Specialist:
- Mechanism: Rifaximin binds to the beta-subunit of bacterial DNA-dependent RNA polymerase, blocking one of the steps in transcription. This results in the inhibition of bacterial protein synthesis and cell growth.
- Zero Absorption Advantage: Unlike standard antibiotics (like Ciprofloxacin) that enter the bloodstream and cause systemic side effects, Rifaximin is < 0.4% absorbed. It stays almost entirely within the gastrointestinal tract, delivering extremely high concentrations of the drug exactly where the infection is, without affecting the rest of the body.
- Microbiome Friendly: It has minimal impact on the beneficial gut flora compared to systemic antibiotics, reducing the risk of post-treatment fungal infections or C. difficile overgrowth.
Product Composition & Strength
We supply this product as Film Coated Tablets (Pink/Red). We strictly utilize the Polymorph Alpha form to ensure non-absorption.
| Active Ingredient | Strength (Standard) | Target Condition | |
|---|---|---|---|
| Rifaximin BP/USP | 200 mg | Traveler’s Diarrhea (TD) | |
| Rifaximin BP/USP | 400 mg | TD / IBS-D | |
| Rifaximin BP/USP | 550 mg | Hepatic Encephalopathy (HE) / IBS-D | |
| Excipients | Q.S. | Sodium Starch Glycolate | Disintegrant |
*Pack Sizes: Blister packs of 10s (Box of 10×10), 14s, or Bulk HDPE Jars (Liver Clinic Packs).
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.20.99 (Medicaments containing antibiotics) |
| Dosage Form | Tablet (Film Coated) |
| Packaging | Alu-Alu Blister (Moisture Sensitive) |
| Storage | Store below 25°C. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Polymorph Control (CRITICAL): Rifaximin exists in various crystal forms (Alpha, Beta, Gamma, etc.). Only Form Alpha is poorly absorbed. Other forms can be absorbed systemically, leading to toxicity. Our QC labs use X-Ray Diffraction (XRD) to certify that every batch is pure Form Alpha.
- Solubility Engineering: Despite being non-systemic, the tablet must disintegrate rapidly to coat the intestinal lining. We use super-disintegrants to ensure dispersion in the duodenum.
Therapeutic Indications (Human Use)
Indicated for gastrointestinal and hepatic disorders:
- Traveler’s Diarrhea (TD): Treatment of TD caused by noninvasive strains of E. coli.
- Hepatic Encephalopathy (HE): Reduction in risk of overt HE recurrence in patients with advanced liver disease (Cirrhosis). It kills the ammonia-producing bacteria in the gut.
- Irritable Bowel Syndrome (IBS-D): Treatment of IBS with diarrhea in adults (improves bloating and stool consistency).
- SIBO: Small Intestinal Bacterial Overgrowth (Off-label use).
Dosage & Administration
Recommended Dosage (Strictly as per Gastroenterologist):
- Route: Oral.
- Traveler’s Diarrhea: 200 mg three times daily for 3 days.
- Hepatic Encephalopathy: 550 mg twice daily (long-term maintenance).
- IBS-D: 550 mg three times daily for 14 days.
- Administration: Can be taken with or without food.
Safety Warnings:
- Systemic Infection: DO NOT USE for diarrhea with fever or bloody stools (Dysentery). Rifaximin stays in the gut and cannot treat infections that have invaded the bloodstream (like Typhoid or Shigella).
- Liver Disease: Safe for liver patients (primary use case), but monitoring is standard.
- Resistance: Cross-resistance to other Rifamycins (like Rifampicin used in TB) is possible but rare.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in