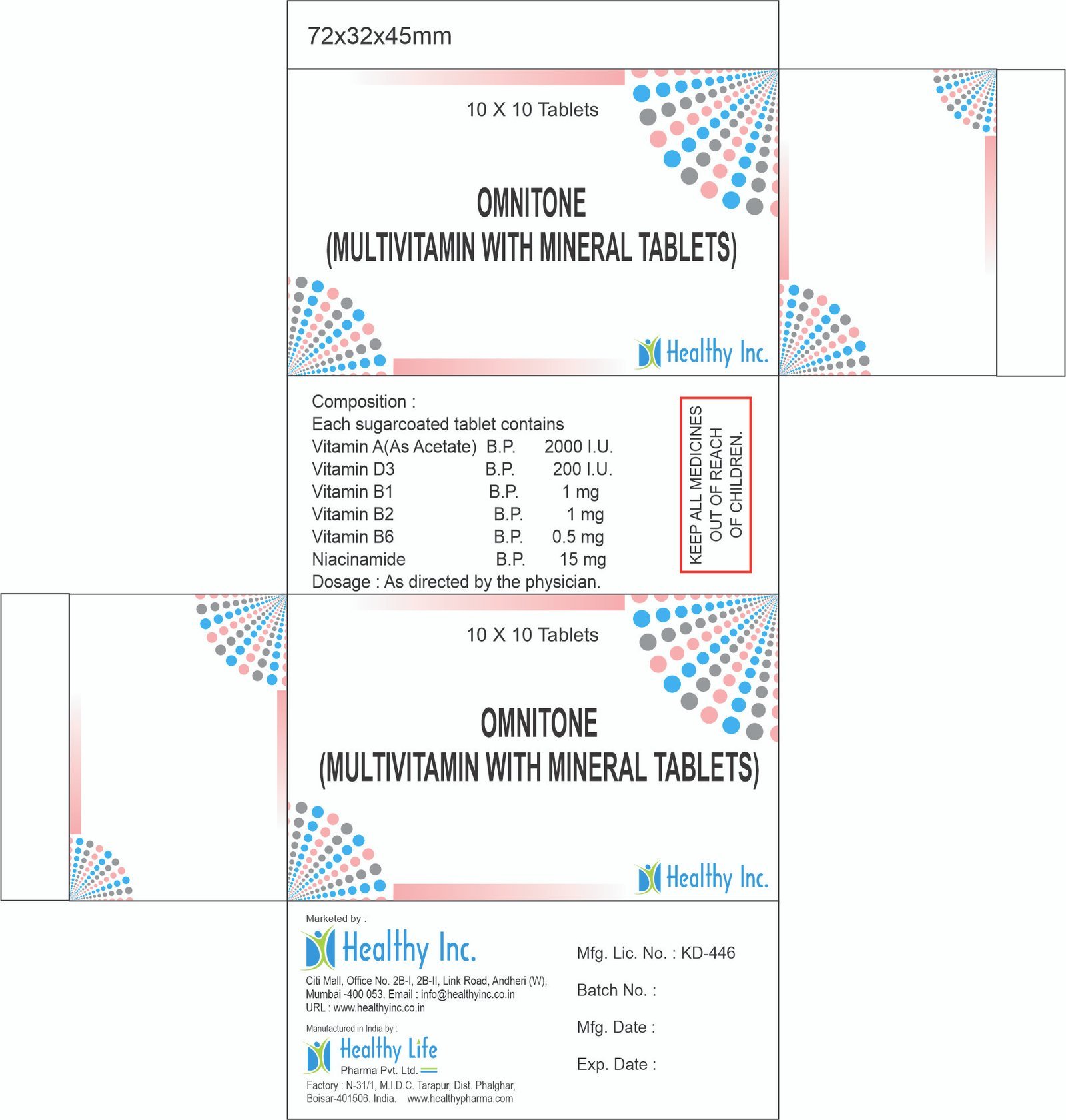
Description
Amiodarone Injection 150mg
Healthy Inc is a specialized global supplier and exporter of critical care antiarrhythmics. We provide high-purity Amiodarone Hydrochloride Injection (150 mg/3 ml), sourced from WHO–GMP certified sterile injectable facilities. This “Cardiac Stabilizer” is a top export to Cardiac Care Units (CCU), emergency departments, and government health ministries in Africa, LATAM, and Southeast Asia, serving as the first-line chemical defibrillator for life-threatening ventricular arrhythmias.
Product Overview
This formulation contains Amiodarone Hydrochloride, a Class III antiarrhythmic agent with a broad spectrum of activity affecting multiple ion channels.
The “Multi-Channel” Blocker:
- Mechanism (Class III Dominant): Its primary action is blocking Potassium (K+) channels, which prolongs the repolarization phase and the refractory period of cardiac cells. This prevents the heart from beating too fast (tachycardia) or irregularly (fibrillation).
- Auxiliary Action (Class I, II, IV): Unlike other drugs, Amiodarone also blocks Sodium channels (Class I), Calcium channels (Class IV), and Beta-adrenergic receptors (Class II). This “broad-spectrum” effect makes it effective against complex arrhythmias where single-channel blockers fail.
- Coronary Vasodilation: It relaxes smooth muscles in the coronary arteries, improving blood flow to the heart muscle during ischemic episodes.
Product Composition & Strength
We supply this product as a Sterile Solution in Amber Glass Ampoules. The formulation utilizes specific co-solvents to solubilize the lipophilic Amiodarone molecule.
| Active Ingredient | Strength (Standard) | Therapeutic Role | |
|---|---|---|---|
| Amiodarone HCl BP/USP | 150 mg / 3 ml (50 mg/ml) | Antiarrhythmic Agent | |
| Vehicle / Solvents | Q.S. | Polysorbate 80 / Benzyl Alcohol | Solubilizing Agents |
*Pack Sizes: Tray of 5 Ampoules or 10 Ampoules.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.90.99 (Other Medicaments) |
| Dosage Form | Injection (Concentrate for Infusion) |
| Packaging | Amber Glass Ampoule (Light Sensitive) |
| Storage | Store below 25°C. Protect from Light. Do not refrigerate (may precipitate). |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Solubility Engineering: Amiodarone is practically insoluble in water. We use a precise ratio of Polysorbate 80 (Tween 80) and Benzyl Alcohol to keep it in solution. This requires strict quality control to prevent “foaming” during filling and ensure the ampoule is free from particulate matter.
- Nitrogen Flushing: The molecule is sensitive to oxidation. Our filling lines use inert nitrogen gas to displace oxygen in the headspace of the ampoule, ensuring stability over the 2-year shelf life.
Therapeutic Indications (Human Use)
Indicated for the management of life-threatening recurrent arrhythmias:
- Ventricular Fibrillation (VF): Resuscitation of cardiac arrest victims resistant to shock therapy (defibrillation).
- Ventricular Tachycardia (VT): Hemodynamically unstable VT.
- Atrial Fibrillation (AFib): Rate control and conversion to sinus rhythm in patients with heart failure.
Dosage & Administration
Recommended Dosage (Strictly as per Cardiologist/ACLS Protocols):
- Cardiac Arrest (VF/Pulseless VT): 300 mg (2 ampoules) IV Push (Rapid). May repeat 150 mg if needed.
- Stable VT/AFib: Loading dose of 150 mg over 10 minutes, followed by a slow infusion (360 mg over 6 hours).
- Dilution (CRITICAL WARNING): Must be diluted in 5% Dextrose (D5W).
- DO NOT dilute in Normal Saline (NaCl). Amiodarone is incompatible with saline and will precipitate (crystallize) immediately.
- Administration: Central Venous Catheter is preferred for infusions to prevent phlebitis.
Safety Warnings (CRITICAL):
- Hypotension: Rapid IV injection causes a sharp drop in blood pressure, primarily due to the Polysorbate 80 / Benzyl Alcohol solvents, not just the drug itself. Administer slowly unless the patient is in cardiac arrest.
- Phlebitis: Causes severe irritation to peripheral veins. Use a central line whenever possible.
- Pulmonary Toxicity: Long-term use can cause lung damage (fibrosis). Acute use is generally safer but monitor oxygenation.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for Sterile Injectables, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.








Reviews
There are no reviews yet.