Description
Doripenem Monohydrate Injection
Healthy Inc is a specialized global supplier and exporter of high-potency carbapenem antibiotics. We provide high-purity Doripenem Monohydrate Injection (500 mg / 250 mg), sourced from WHO–GMP certified sterile injectable facilities. This “Critical Care Precision” agent is a top export to intensive care units (ICU), urology departments, and government health ministries in Africa, LATAM, and Southeast Asia, serving as the most potent anti-pseudomonal carbapenem for treating complicated intra-abdominal and urinary tract infections.
Product Overview
This formulation contains Doripenem Monohydrate, a synthetic broad-spectrum carbapenem antibiotic structurally related to Meropenem.
The “Pseudomonas” Specialist:
- Enhanced Potency: Doripenem exhibits superior in-vitro activity against *Pseudomonas aeruginosa* compared to Meropenem and Imipenem. It has a higher affinity for specific Penicillin-Binding Proteins (PBPs) in Pseudomonas, making it the drug of choice for resistant strains in ICUs.
- Seizure Safety: Unlike Imipenem (which carries a risk of seizures), Doripenem has a low epileptogenic potential, similar to Meropenem, making it safer for patients with neurological disorders or renal impairment.
- Stability (Prolonged Infusion): It is highly stable in solution, allowing for extended infusions (up to 4 hours). This pharmacokinetic advantage maximizes the time the drug concentration remains above the MIC (Time > MIC), which is critical for killing resistant Gram-negative bacteria.
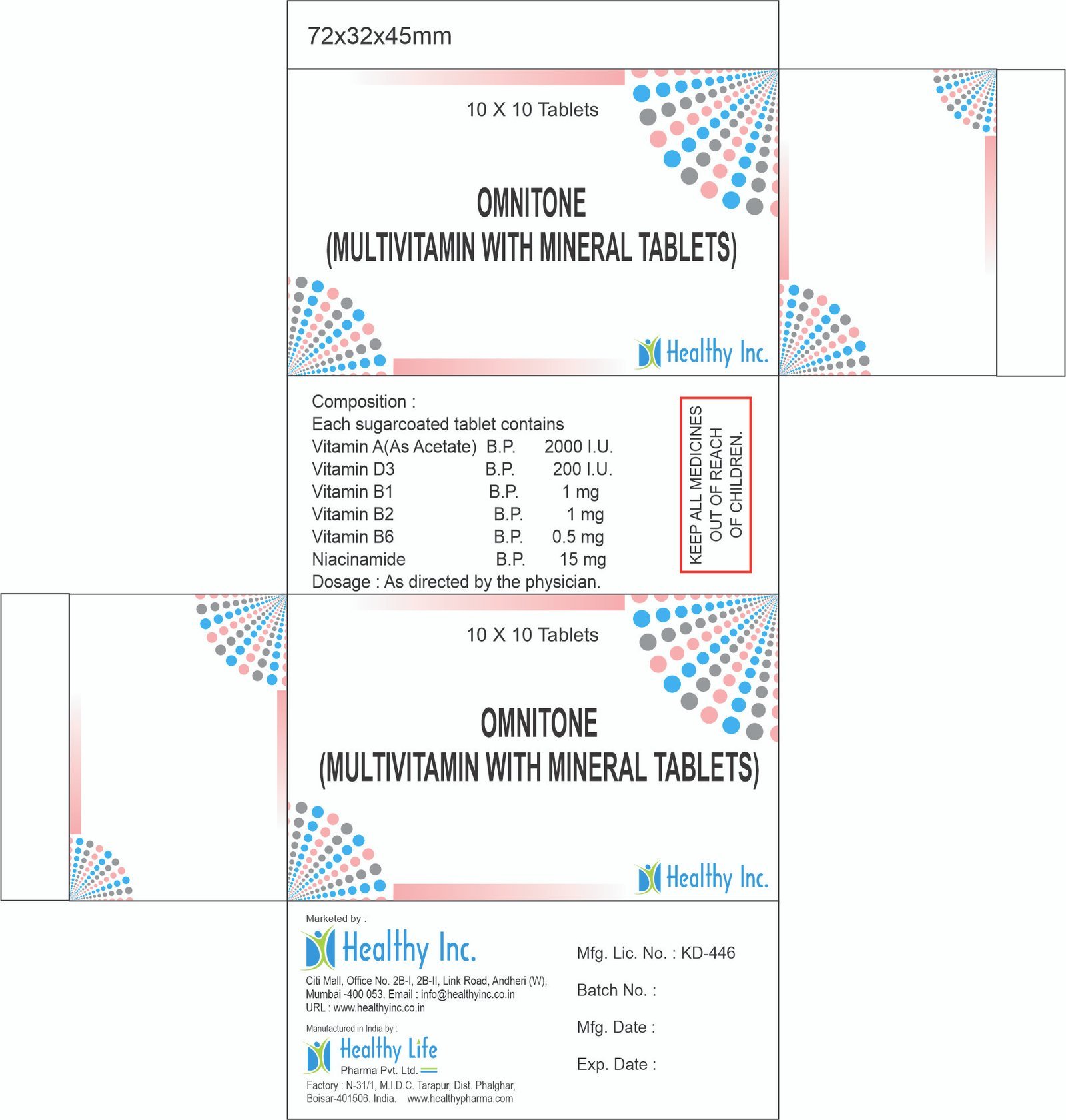
Product Composition & Strength
We supply this product as a Sterile Lyophilized Powder for Injection in glass vials.
| Active Ingredient | Strength | Primary Use |
|---|---|---|
| Doripenem Monohydrate USP/BP | Equivalent to 500 mg Doripenem | Standard Adult Dose |
| Doripenem Monohydrate USP/BP | Equivalent to 250 mg | Renal Impairment |
| Excipients | None (Pure Sterile Powder) | – |
*Pack Sizes: Tray of 1 Vial, 10 Vials, or Box of 1/10 Vials.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.20.19 (Medicaments containing other antibiotics) |
| Dosage Form | Lyophilized Powder (Reconstitute & Dilute) |
| Packaging | Type I Glass Vial (Sterile) with Flip-off Seal |
| Storage | Store below 25°C. Protect from Light. Reconstituted solution stable for 12 hours at room temp (if diluted in Saline). |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Crystallization Stability: Doripenem is difficult to crystallize purely. We use a proprietary sterile crystallization process that ensures a stable polymorph, preventing degradation during shelf life and ensuring rapid dissolution (reconstitution) in the hospital pharmacy.
- Low Endotoxin: Given its use in septic patients, we strictly control bacterial endotoxin levels to be well below limits, minimizing the risk of fever or shock upon administration.
Therapeutic Indications (Human Use)
Indicated for the treatment of serious bacterial infections:
- Complicated Intra-Abdominal Infections (cIAI): Appendicitis, cholecystitis, diverticulitis, and peritonitis (covers anaerobes and aerobes).
- Complicated Urinary Tract Infections (cUTI): Including Pyelonephritis caused by ESBL-producing E. coli or Klebsiella.
- Nosocomial Pneumonia (Off-label/Guideline-based): Ventilator-associated pneumonia (VAP) caused by non-fermenting Gram-negatives (Pseudomonas/Acinetobacter).
Dosage & Administration
Recommended Dosage (Strictly as per Critical Care Physician):
- Route: Intravenous (IV) Infusion ONLY.
- Standard Dose: 500 mg every 8 hours.
- Infusion Time: Administer over 1 hour (Standard) or 4 hours (Extended Infusion) for severe/resistant infections.
- Renal Impairment:
- CrCl 30-50 ml/min: 250 mg every 8 hours.
- CrCl 10-30 ml/min: 250 mg every 12 hours.
- Reconstitution: Dissolve 500mg in 10ml WFI/Saline. Dilute further in 100ml Saline.
Safety Warnings (CRITICAL):
- Valproic Acid Interaction: Carbapenems reduce serum Valproic Acid levels by 60-100%, leading to breakthrough seizures. Avoid co-administration in epileptic patients.
- Penicillin Allergy: Cross-reactivity exists. Use with caution in patients with history of anaphylaxis to beta-lactams.
- Pneumonia Mortality: In clinical trials, Doripenem showed higher mortality in VAP compared to Imipenem/Cilastatin if used for 7 days. Ensure appropriate duration (10-14 days) and combination therapy for Pseudomonas.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for Sterile Injectables, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.






Reviews
There are no reviews yet.