Description
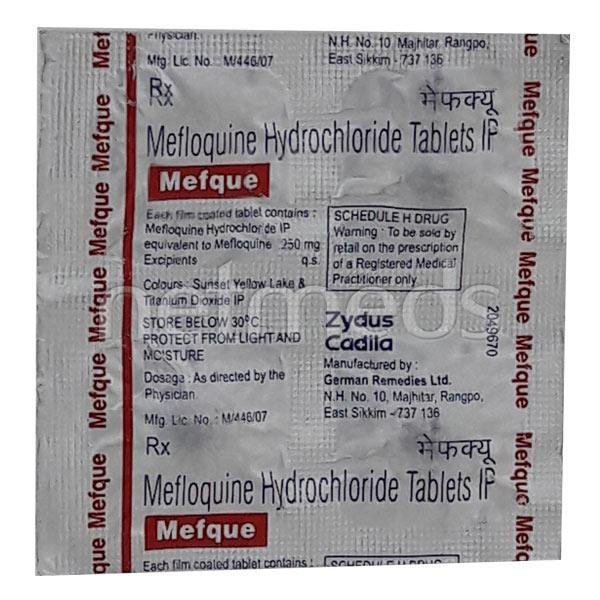
Mefloquine Hydrochloride Tablets (250 mg)
Healthy Inc is a specialized global supplier of high-stability antimalarial therapies. We provide premium Mefloquine Tablets, manufactured in WHO–GMP certified antimicrobial facilities. As a critical agent for both the prophylaxis and treatment of resistant malaria, this product is a primary export for travel clinics, military health units, and Ministry of Health (MOH) tenders in Southeast Asia, Sub-Saharan Africa, and Latin America.
Product Overview
Mefloquine is a synthetic 4-quinolinecarbinol derivative. It is specifically engineered to combat strains of Plasmodium falciparum that have developed resistance to chloroquine and other traditional antimalarials, serving as a vital tool in global malaria control programs.
The “Resistant Strain” Specialist:
- Mechanism of Action: Mefloquine acts as a blood schizonticide. It is believed to interfere with the parasite’s ability to detoxify heme (a byproduct of hemoglobin digestion), leading to a buildup of toxic heme complexes that destroy the parasite from within.
- Long-Lasting Prophylaxis: Due to its exceptionally long elimination half-life (approx. 2 to 3 weeks), Mefloquine is one of the few medications suitable for once-weekly dosing in travelers visiting high-risk malaria zones.
- Broad Efficacy: It is highly effective against P. falciparum and P. vivax, including multi-drug resistant (MDR) strains found in the Southeast Asian “malaria belt.”
Technical & Manufacturing Specifications
Formulated for high chemical stability and consistent dissolution in tropical environments.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Mefloquine Hydrochloride BP / USP / IP |
| Dosage Form | Scored Tablets (for flexible dosing) |
| Assay (Purity) | 99.0% – 101.0% |
| HS Code | 3004.20.19 (Antimalarials) / 2933.49.00 |
| Stability | Validated for Zone IVb (High heat/humidity) export conditions |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from ISO 9001:2015 certified units.
- Precision Scored Design: Our tablets are designed with a cross-score to allow for accurate pediatric dosing and fragmented administration, ensuring therapeutic flexibility in field conditions.
- Moisture Protection: Mefloquine can be susceptible to environmental degradation. We utilize Alu-Alu packaging to ensure the tablets maintain 100% potency in tropical rainforest and sub-Saharan climates.
- Bioavailability Consistency: Every batch undergoes comparative dissolution testing to ensure it matches the absorption kinetics of the innovator brand (Lariam), ensuring predictable clinical outcomes.
Therapeutic Indications & Clinical Symptoms
- Malaria Prophylaxis: Prevention of malaria in travelers visiting areas where chloroquine-resistant P. falciparum is prevalent.
- Acute Treatment: Management of uncomplicated malaria caused by P. falciparum or P. vivax.
- MDR Malaria: Use in regions with known resistance to multiple other antimalarial classes.
Dosage & Administration
- Prophylaxis: 250 mg once weekly, starting 1-2 weeks before travel and continuing for 4 weeks after leaving the endemic area.
- Treatment: A total dose of 1250 mg, usually administered in divided doses (e.g., 750 mg followed by 500 mg 6–12 hours later) to minimize GI side effects.
- Safety Note: Contraindicated in patients with active or a history of psychiatric disorders or seizures. Patients should be monitored for neuropsychiatric side effects.
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Pharmaceutical Exporter in Mumbai, catering to International Aid Agencies, Military Supply Chains, and Ministry of Health Tenders. We offer Third Party Manufacturing for Mefloquine with full CTD Dossier and COPP support. Our logistics network ensures streamlined delivery to Vietnam, Nigeria, Brazil, and Thailand, providing WHO-GMP quality at competitive B2B prices.
Commercial Inquiries
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in