Description
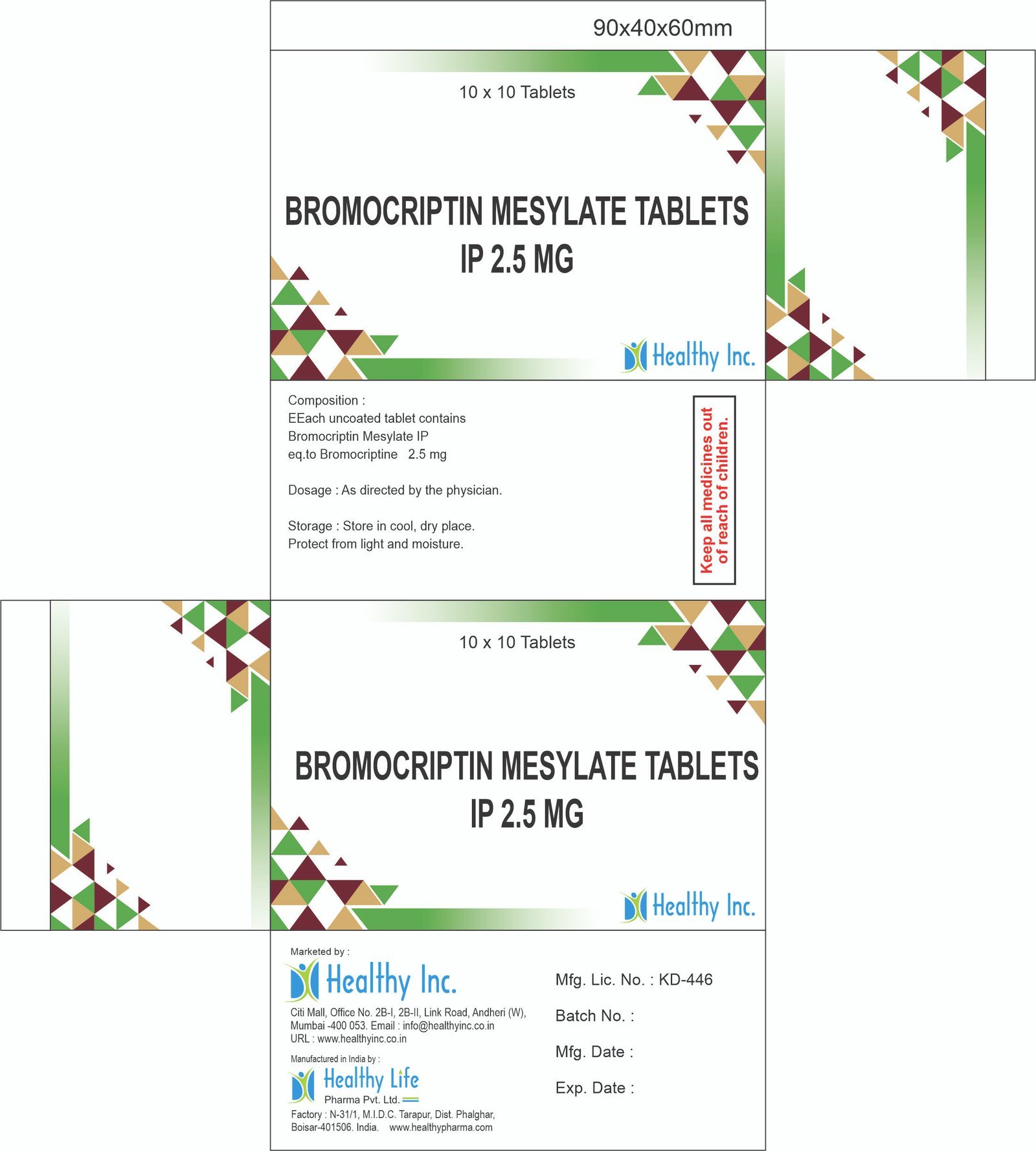
Leflunomide Tablets (10 mg / 20 mg / 100 mg)
Healthy Inc is a specialized global supplier of high-performance immunomodulatory therapies. We provide premium Leflunomide Tablets, manufactured in WHO–GMP certified specialized potent-drug units. Designed to arrest the progression of autoimmune joint destruction, this product is a cornerstone export for rheumatology clinics, rehabilitation centers, and specialized hospital tenders across Southeast Asia, Africa, and Europe.
Product Overview
Leflunomide is a synthetic isoxazole derivative and a potent Disease-Modifying Antirheumatic Drug (DMARD). It is specifically engineered to target the underlying inflammatory process of autoimmune diseases by selectively inhibiting the proliferation of activated lymphocytes.
The “Structural Preservation” Specialist:
- Mechanism of Action: Leflunomide is a prodrug that is rapidly converted to its active metabolite, Teriflunomide (A77 1726). It inhibits the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), which plays a pivotal role in the de novo synthesis of pyrimidines. Since activated T-cells depend on this pathway for expansion, Leflunomide effectively limits the autoimmune attack on joint tissues.
- Clinical Efficacy: It is clinically proven to reduce signs and symptoms of Rheumatoid Arthritis (RA), inhibit structural damage (as evidenced by X-ray erosions), and improve physical function.
- Psoriatic Arthritis: Beyond RA, it is an essential therapy for managing the skin and joint manifestations of active Psoriatic Arthritis.
Technical & Manufacturing Specifications
Formulated with specialized containment protocols for high-potency active ingredients.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Leflunomide BP / USP / IP |
| Dosage Form | Film-Coated Tablets |
| Standard Strengths | 10 mg, 20 mg (Maintenance) / 100 mg (Loading) |
| HS Code | 3004.90.99 (Medicaments) / 2934.99.90 |
| Packaging | Alu-Alu Blister (Crucial for chemical stability and light protection) |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from specialized ISO 9001:2015 units.
- Containment Excellence: Due to its immunomodulatory nature, Leflunomide is manufactured in segregated facilities with advanced air filtration (HEPA) to prevent cross-contamination, ensuring absolute product purity.
- Stability Validation: Validated for Climatic Zone IVb, our tablets utilize a specialized moisture-barrier film coating that ensures the isoxazole ring remains intact for 36 months in tropical and humid environments.
- Dissolution Precision: Utilizing advanced wet granulation, we ensure that the tablet releases the API consistently, matching the pharmacokinetic profile required for steady-state plasma concentrations.
Therapeutic Indications & Clinical Symptoms
- Rheumatoid Arthritis (RA): Treatment of active RA in adults to reduce joint swelling, stiffness, and pain.
- Psoriatic Arthritis: Management of active psoriatic arthritis to improve joint mobility and skin condition.
- Off-Label Use: Sometimes utilized in specialized transplant medicine or severe systemic lupus erythematosus (SLE) under expert supervision.
Dosage & Administration
- Loading Dose: Historically 100 mg once daily for 3 days (often omitted now to reduce side effects).
- Maintenance Dose: 10 mg to 20 mg once daily.
- Safety Note: Leflunomide has a long half-life. It is highly teratogenic; a strict “Washout Procedure” using Cholestyramine is required for patients wishing to conceive. Regular monitoring of ALT/AST (Liver enzymes) and Blood Pressure is mandatory.
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Pharmaceutical Exporter in Mumbai, catering to Rheumatology Centers and National Health Tenders. We offer Third Party Manufacturing for Leflunomide with full CTD Dossier and COPP support. Our logistics network ensures secure transit to Vietnam, Nigeria, UK, and the Philippines, providing WHO-GMP quality at competitive B2B prices.
Commercial Inquiries
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in