Description
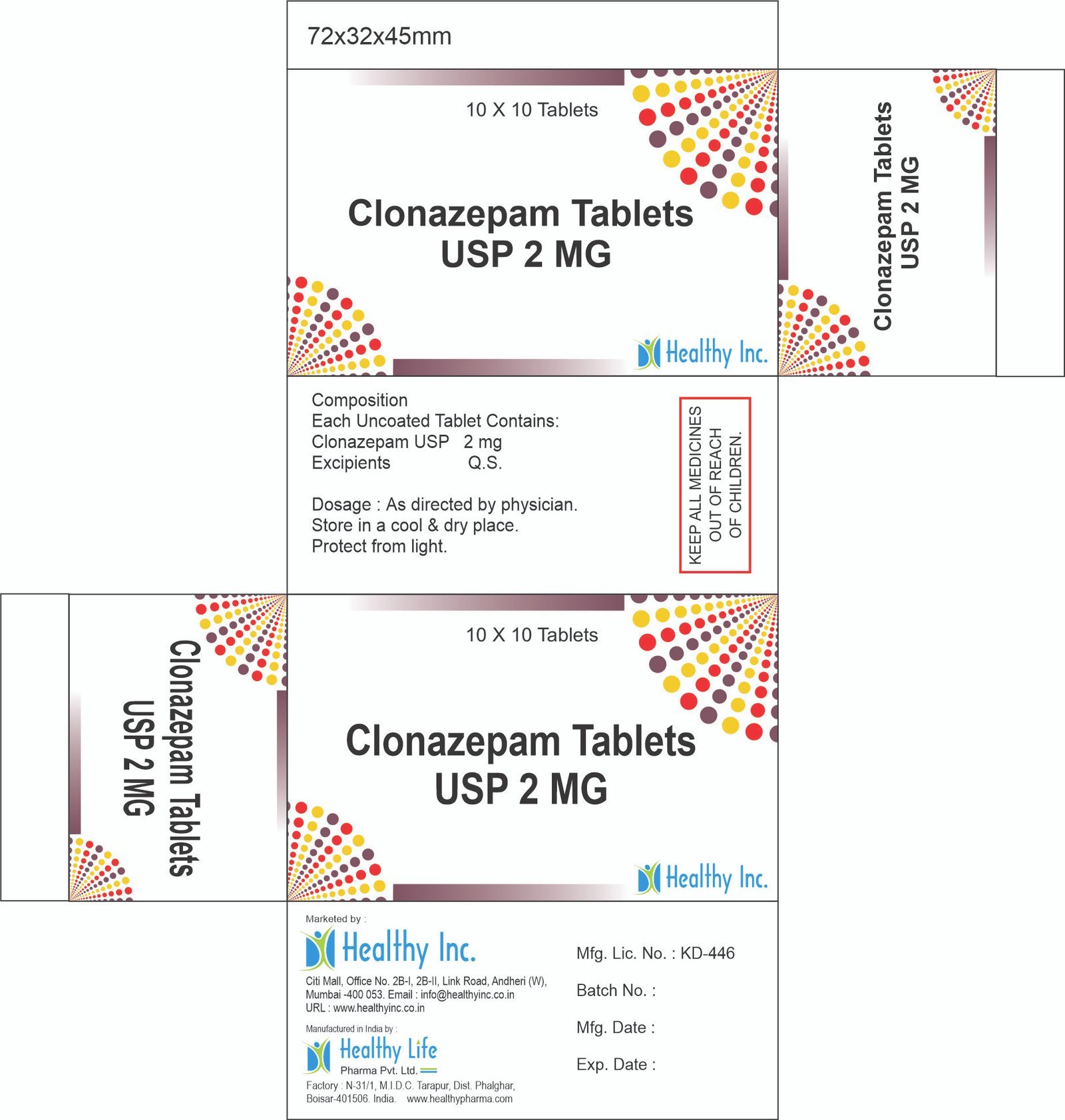
Clonazepam Tablets (0.5 mg / 1 mg / 2 mg)
Healthy Inc is a premier global supplier of high-precision neuropsychiatric therapies. We provide premium Clonazepam Tablets, manufactured in WHO–GMP certified specialized psychotropic production units. As a long-acting benzodiazepine essential for seizure control and panic disorder management, this product is a vital export for neurological institutes, psychiatric hospitals, and Ministry of Health (MOH) tenders in Southeast Asia, Africa, and the Middle East.
Product Overview
Clonazepam is a chlorinated derivative of nitrazepam and a potent anticonvulsant. It is specifically engineered to suppress the spike-and-wave discharge in absence seizures and decrease the frequency, amplitude, and duration of minor motor seizures by enhancing the inhibitory neurotransmission of GABA.
The “Neural Stabilizer” Specialist:
- Mechanism of Action: Clonazepam acts as a positive allosteric modulator of the GABA-A receptor. By increasing the frequency of chloride channel opening, it hyperpolarizes the postsynaptic neuron, leading to a profound inhibitory effect on the central nervous system (CNS).
- Broad Anticonvulsant Spectrum: It is highly effective in the management of Lennox-Gastaut syndrome, akinetic, and myoclonic seizures, as well as being a primary treatment for Panic Disorder with or without agoraphobia.
- Pharmacokinetic Longevity: With an intermediate onset and a long half-life (30–40 hours), Clonazepam provides sustained symptomatic relief and stable plasma concentrations, reducing the risk of inter-dose withdrawal symptoms.
Technical & Manufacturing Specifications
Formulated for absolute content uniformity in micro-dose strengths.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Clonazepam BP / USP / IP |
| Dosage Form | Immediate Release Tablets / Mouth Dissolving (MDT) |
| Available Strengths | 0.25 mg, 0.5 mg, 1 mg, 2 mg |
| HS Code | 3004.90.72 (Psychotropic Medicaments) / 2933.91.00 |
| Packaging | Alu-Alu Blister (Crucial for light-sensitive benzodiazepines) |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from ISO 9001:2015 certified units.
- Controlled Substance Compliance: Our facilities are licensed for the production of Schedule IV / Class II psychotropic substances, ensuring rigorous international tracking and narcotic export documentation (INCB standards).
- Micro-Dose Precision: Handling low-dose APIs (0.25mg/0.5mg) requires Geometric Dilution and high-shear granulation. We guarantee 100% content uniformity to ensure patient safety and prevent sub-therapeutic dosing.
- Stability Validation: Validated for Zone IVb climates. Our tablets utilize specialized binders to prevent crumbling and maintain disintegration profiles in high-humidity regions.
Therapeutic Indications & Clinical Symptoms
- Seizure Disorders: Treatment of absence seizures (petit mal), myoclonic seizures, and status epilepticus (refractory).
- Panic Disorder: Reduction of acute panic attacks and anticipatory anxiety.
- Movement Disorders: Management of akathisia and certain types of dystonia.
- Bipolar Disorder: Adjunctive treatment for acute mania or sleep disturbances.
Dosage & Administration
- Initial Dose: Typically 0.25 mg to 0.5 mg twice daily, slowly titrated to avoid excessive sedation.
- Maximum Dose: Generally does not exceed 4 mg/day for panic disorder or 20 mg/day for epilepsy.
- Safety Note: High risk of dependence. Avoid abrupt discontinuation to prevent withdrawal seizures. Contraindicated in patients with significant liver disease or acute narrow-angle glaucoma.
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Psychotropic Drug Exporter in Mumbai, catering to Neurology Centers and Ministry of Health Tenders. We offer Third Party Manufacturing for Clonazepam with full CTD Dossier and international narcotic permit support. Our logistics network ensures secure transit to Vietnam, Nigeria, UAE, and the Philippines, providing WHO-GMP quality at competitive B2B prices.